Is Atkins Dead (Again)?
Nutrition, Metabolism and Cardiovascular Disease 14(2004):61.
by Michael E.J. Lean, M.D., F.R.C.P., F.R.C.P.S.
Professor Lean, head of the Department of Human Nutrition, holds the position of Rank Chair of Human Nutrition at the University of Glasgow where he is also a Consultant Physician at Glasgow Royal Infirmary. He trained in medicine at the University of Cambridge and St Bartholemew’s. In a research capacity he has over 300 publications to his name.
Abstract
Despite consistent epidemiological evidence that weight gain is linked to higher fat and lower carbohydrate consumption, supported by animal evidence and the inescapable truth that fat supplies 9 kcal / g compared to 3.75 kcal / g from carbohydrates, low-carbohydrate “Atkins” style diets are heavily promoted for obesity control. The randomised controlled trial evidence is very small. The totality of the evidence continues to show that low-carbohydrate diets are marginally disadvantageous for long-term health and for weight maintenance. People can lose weight equally well on low-carbohydrate (“Atkins-style”) diets, and some groups of obese patients tend to lose a little more than on high-carbohydrate groups. This small difference (1-2 kg) may be explained by rapid loss of (glycogen-associated) body water, or by the influence of extraordinary media coverage leading to elevation of expectation and compliance with low-carbohydrate diets in the short term.
Body
Two papers were published in the same edition of New England Journal of Medicine in May 2003 (1, 2). Both reported very similar studies, with similar results, in attempts to establish by randomised controlled trials whether obese patients would benefit more from a slimming diet with low carbohydrate or low fat content. The level of interest in this issue has resulted from publicity given to the “Atkins” carbohydrate restriction which has achieved fashion-cult status amongst society figures but little support from dietitians, doctors or nutritional scientists who were already familiar with previously published research on benefits of low-fat diets which is not quoted by the “Atkins” regimen.
Foster et al overtly tested the “Atkins” method (< 20g/d carbohydrate without limiting fat or protein using the Atkins handbook) compared to a conventional diet (60% Energy carbohydrate 25% Energy fat, using the well-known “Learn” handbook of Brownell) (3). Sixty-three patients started, but only 49 continued for three months, and 37 for 12 months. Drop outs were more frequent (30%) on the conventional diet at three months, but about 40% had given up on both diets at 12 months, so information about weight maintenance on the Atkins diet can only be based on 20 subjects at 12 months. No information is provided on physical activity or exercise patterns before and during the study.
Weight loss at three months was 6.8% body weight (SD 5.0) on the Atkins diet which were significantly better than 2.7 (SD 3.7) kg loss on the conventional diet. At 12 months there was no longer a difference in weight change between the two diets. The greater weight loss was accompanied by significantly better lipid profiles on the Atkins diet at three months, but metabolic differences were minimal at 12 months. The authors conclude that a larger, longer trial would be necessary, to be confident about differences which means that any differences must be small or restricted to rather few patients. The high standard deviations show how variable the responses are. Perhaps that means that RCT is not the best way to test diets. Allocating patients to a diet they do not like would be likely to result in many dropouts and variable responses.
The second paper did not follow the principles of Atkins completely, but randomised 132 obese metabolic syndrome patients to either low carbohydrate (£ 30g/day) or low fat (<30% dietary energy) (1). In this study authors stated, “No specific exercise program was recommended”. Even more patients dropped out of this trial – only half in the low fat group, and two-thirds in the low CHO continued to six months. At that stage weight losses were greater in the low CHO group 5.8 (SD 8.6) kg vs 1.9 (SD 4.2) kg and similar figures were found when dropouts were included assuming no weight loss. No longer term data relevant to weight maintenance were collected.
There were improvements in lipid variables and insulin sensitivities with weight loss and the lipid changes on the low CHO diet remained even after adjustment for the weight change.
With any study of free-living subjects, compliance is an issue. A problem with the study of Samaha et al (1) was that the reported baseline fat consumption of these subjects was 33%E (with 51% CHO) and after six months on the supposedly low-fat diet these figures were completely unchanged. There was a reported change to 41%E from fat, 37%E from carbohydrate, on the low-CHO diet. Thus the only change in diet composition was with the low CHO diet advice. These were older subjects (54 SD 9 years), a factor which may have played a part in compliance. The role of previous dietary advice and beliefs on compliance cannot be assessed. Foster et al (2) measured urinary ketones and found them more often in those on the Atkins (ketogenic) diet – but in only 40-60% at 1-3 months and only 10-20% between 3-12 months, while in 5-10% of those supposed to be on the high carbohydrate diet also showed urinary ketones. These results point to wide and overlapping ranges of dietary behaviours. People probably pick their own slimming diet for short-term weight loss, largely irrespective of the specific dietary advice given.
The low carbohydrate, high protein dietary approach promoted (for commercial reasons) by Atkins is loosely tethered to strands of evidence on the effects of nutrients on insulin release and action, but the theory base is incomplete and does not acknowledge the (inconvenient) totality of the evidence. For example, carbohydrate stimulates insulin – which promotes lipogenesis - but many amino acids are also potent insulin secretagogues. This theoretical evidence would also support the use of low-glycaemic index diets for weight management, and there is growing evidence to support the use of more liberal carbohydrate with low glycaemic index for weight loss (4, 5). Advice to restrict carbohydrate severely leads to a diet with relatively high proportions of energy coming from fat and protein. Protein satisfies appetite and is mostly metabolised in the same way as carbohydrates. The amount of protein does not appear enough to upset renal function in these studies. However, because so much fat in Western foods is saturated, people following an Atkins-style diet can end up with high consumptions of saturated fat – particularly after the period of energy restriction and weight loss has finished. If that is the case, then elevation of LDL cholesterol and atherogenic index will occur. There was vigorous debate on this issue in the context of “diabetic diets” in the 1980s. Prominent American researchers consistently found better metabolic profiles with low-carbohydrate (higher fat) diets than with high-carbohydrate (lower fat) diets (6, 7). Several European groups found consistently better metabolic profiles with high-carbohydrate (low-fat) diets (8, 9). This debate was ultimately settled amicably with the recognition that American carbohydrates tended to be more simple sugars (including fructose which tends to elevate serum triglycerides), and American fats tend to contain more MUFA and less saturated fats. European carbohydrates contained less sugars and more soluble dietary fibre, which served to counteract the potential hazard of higher carbohydrate consumption. This would now be recognised as one aspect of a low glycaemic index diet.
Another potential hazard from a low carbohydrate diet is a relative depletion of the micronutrients found in high (European) carbohydrate foods (table 1). Many high carbohydrate foods are particularly rich in antioxidant vitamins and minerals as well as thiamine (Vit B1), pyridoxine (Vit B6) and Folate. Many people, especially teenage girls are low or marginal for these nutrients in Western countries. Restricting these foods could aggravate sub-clinical deficiencies to cause long-term ill health, without this necessarily being detectable in blood tests. It is an unfortunate feature of Human Nutrition that functional problems often develop with dietary deficiencies before there are detectable changes in blood biochemistry.
Deficiency of antioxidants and folic acid would tend to accelerate CHD, by allowing LDL oxidation and increasing blood homocysteine respectively (10, 11).
Weight management has three related components which need to be considered separately, and which in principle may need different treatments:
1. Long term: prevention of weight (re) gain
This is essentially a cure for the disease process of obesity. It applies both before unacceptable fat accumulation has developed and after weight loss.
2. Short term: weight loss
Energy restriction for active weight loss should be limited to 5-6 months, and always followed by prevention of weight regain.
3. Treatment of coexisting CHD risk factors
The hazards from conventional risk factors for CHD (smoking, type 2 DM, hypertension, hyperlipidaemia) are all greatly exaggerated in the overweight, so should be prioritised for treatment (12)
The balance of fat, protein and carbohydrate in the diet has been known for many years to affect all of these components.
1. Long term weight maintenance and avoidance of weight gain depends on limiting energy intake to match needs. Achieving this balance is much easier for people who are physically active. Modest exertion – such as walking promotes a feeling of satiety, burns up some calories (about an extra 300 per hour) and does not stimulate subsequent appetite – although it may make food taste nicer (13). People who are physically active tend to choose meals which are higher in carbohydrates than inactive people (14). This is an advantage because carbohydrates contain less than half as many calories per gram or per mouthful as fat (3.75 vs 9.0 kcal/g). Energy dense foods tend to be high in fat, whereas high CHO foods tend to be bulky and fibre-rich. Carbohydrates satisfy appetite more readily than fat. Carbohydrates also stimulates metabolic rate, whereas fat does not (15). The theoretical evidence thus heavily favours a high-carbohydrate, low-fat approach to avoid weight (re) gain. In practice, it is difficult to test this principle because of the difficulty obtaining accurate, truthful information about what people with weight problems eat.
Kelly West showed, many years ago, how populations with high carbohydrate diets tend to stay thinner (16). There were many possible socio-economic confounders in this ecological evidence, but there was certainly no evidence for the opposite effect. Setting aside how much people say they eat, there have been consistent trends in post-industrial and developing countries alike to conclude that the rise in obesity has followed a gradual increase in fat and fall in carbohydrate consumption. Recent economic analyses in USA show how food supply, especially added-fats and sugars, has risen over the last 30 years (17, 18). These studies concluded that the obesity epidemic was initiated – between 1970-90 by falling physical activity, but fuelled by an inappropriate increase in food consumption subsequently. Provided with free choice, people eat more calories if the available foods are high in fat than if they are high in carbohydrates (19). The remarkable study of Weststrate et al provided rare evidence that switching to low-fat foods without conscious food restriction, was enough to prevent most of the weight gain which otherwise “normally” occurs on high-fat European diets (20). Wing and Hill reported that weight loss maintenance is more successful on high-carbohydrate than low-carbohydrate diet (21).
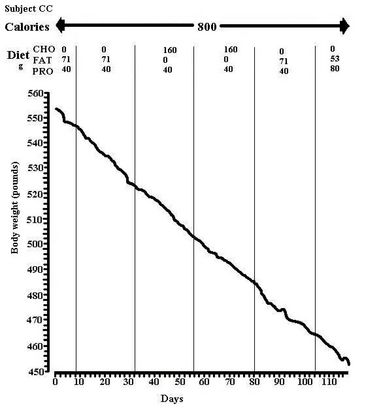
2. Weight loss – or slimming – is almost always limited to 3-4 months in the real world, and so to 5-10 kg loss for most people using ordinary foods. Physical activity is rarely sufficient to generate a big enough energy deficit, so conscious food restriction is necessary. It often helps short-term compliance to follow unusual diets (grapefruit diet, cabbage-soup diet, etc.) but in this context, “a calorie is a calorie” and there is little evidence that advice to follow a relatively low or `relatively high carbohydrate slimming diet will affect the rate of weight loss. This was demonstrated rather clearly many years ago by Bortz et al in inpatients following a 800 kcal/d diet (figure 1) (22, 23). Golay et al drew similar conclusions from carefully controlled studies under metabolic ward conditions (24). Alford et al found no differences between diets which varied in carbohydrates and other nutrients (25). In free-living subjects, where compliance is the main issue, Lean et al studied 110 women for six months, randomised to high (58%) or low (35%) carbohydrate diets of 1200 kcal/d (26). There was no difference between diets in dropout rates (25% at six months) or in the weight loss using either completers or “intention to treat” analysis. Blood pressure and lipids improved with weight loss but there were no differences between diets in any cardiovascular risk factor changes. However, only on the high carbohydrate diet were there improvements from baseline at six months (serum total cholesterol and HDL).
All these studies have shown high variances in weight change, indicating that some subjects have done particularly well on the one diet or the other, with a consistent hint that low carbohydrate diets lead to greater loss in the early stages. Compliance is an over-riding issue dependent on attitudes and beliefs. Lean et al explored this in relation to age by studying a carefully matched sub-sample of post-menopausal women (chosen because they would not have menstrual fluid shifts, but also because their older beliefs about “fattening” foods might make them more sympathetic to carbohydrate restriction (26). In this sub-sample of 46 older subjects there was significantly greater loss on the lower carbohydrate diet for up to 12 months (mean difference 3.5 kg less).
Overview and Conclusions
Viewing the totality of this evidence, it seems that the physiological differences between low and high carbohydrate diets are small during a period of weight loss. At the present time, perhaps influenced by positive publicity for the Atkins diet, the evidence is for marginally greater loss by subjects randomised to low carbohydrate diets. This is mainly a compliance issue. Both diets have high variance between individuals. Depletion of carbohydrate (glycogen) stores on a low CHO diet is likely, with consequent loss of 1-2 kg of body water (27). This non-fat effect would only be demonstrated in very large studies because of the large variance in weight changes. For long-term weight maintenance, and prevention of re-gain, some people feel more comfortable with low carbohydrate (Atkins style) diets, and so tend to do better. These are likely to be older, relatively inactive people: depletion of body glycogen stores would be problematic for people who want to be active.
For reduction of the cardiovascular risks of overweight people, weight loss and maintenance by any route is effective. If an individual is able to do much better on a low CHO diet, then the overall risk factor improvement, with particular fall in triglycerides may outweigh the benefits for total cholesterol of a higher CHO, low-fat diet. Whatever the carbohydrate intake, obese patients are at exaggerated cardiovascular risk from the conventional risk factors (12) so reducing saturated fat and maintaining physical activity have particular value. If individuals can maintain moderate activity (10,000 steps / day) (28), then the choice of diet composition may be less important (29).
Atkins is clearly not dead, and has led us to review our clinical advice, and stimulated new thoughts. There are some individuals – perhaps those with natural predominance of type-1 (oxidative) muscle fibres – who may be less dependent on dietary carbohydrates. At a population level, however, most people with weight problems seem to have relatively high fasting RQ, probably indicating a greater capacity to use stored carbohydrate (or limited capacity to oxidise fat) (30). For the primary prevention of obesity, then, most people would still be advised to follow, or provided with, a relatively high carbohydrate, low glycaemic index (high soluble fibre) low fat diet. Kelly West (16) is not dead either!
References
- Samaha FF, Nayyar I, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L (2003) A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348: 2074-2081.
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed S, Szapary PO, Rader DJ, Edman JS, Klein S (2003) A randomised trial of a low-carbohydrate diet for obesity. N Engl J Med 348: 2082-2090
- Brownell KD. (2000) The LEARN program for weight management 2000. Dallas: American Health Publishing,
- Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. (2003) A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med 157: 773-9.
- Brand-Miller JC, Holt SH, Pawlak DB, McMillan J (2002) Glycemic index and obesity. Am J Clin Nutr 76 :281S-5S.
- Coulston AM, Liu GC, Reaven GM. (1983) Plasma glucose, insulin and lipid responses to high-carbohydrate low-fat diets in normal humans. Metabolism. 32: 52-56.
- Coulston AM, Hollenbeck CB, Swislocki AL, Chen YD, Reaven GM. (1987) Deleterious metabolic effects of high-carbohydrate, sucrose-containing diets in patients with non-insulin-dependent diabetes mellitus. Am J Med. 82: 213-20.
- Simpson HC, Simpson RW, Lousley S, Carter RD, Geekie M, Hockaday TD, Mann JI. (1981) A high carbohydrate leguminous fibre diet improves all aspects of diabetic control. Lancet 1: 1-5.
- Riccardi G, Rivellese A, Pacioni D, Genovese S, Mastranzo P, Mancini M. (1984) Separate influence of dietary carbohydrate and fibre on the metabolic control in diabetes. Diabetologia. 26: 116-21.
- Nakano E, Higgins JA, Powers HJ (2001) Folate protects against oxidative modification of human LDL. Br J Nutr 86: 637-9.
- Samman S, Sivarajah G, Man JC, Ahmad ZI, Petocz P, Caterson ID (2003) A mixed fruit and vegetable concentrate increases plasma antioxidant vitamins and folate and lowers plasma homocysteine in men. J Nutr. 133: 2188-93.
- Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rossner B, Monson RR, Speizer FF, Hennekens CH (1990) A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med 322: 882-889
- Lluch A, King NA, Blundell JE (1998) Exercise in dietary restrained women: no effect on energy intake but change in hedonic ratings. Eur J Clin Nutr 52: 300-307
- Wood PD, Haskell WL, Terry RB (1982) Effects of a two-year running program on plasma lioproteins, body fat and dietary intake in initially sedentary men. Med Sci Sports Exerc 14: 104
- Lean MEJ, James WPT (1988) Metabolic effects of isoenergetic nutrient exchange over 24 hours in relation to obesity in women. Int J Obes 12: 15-28
- West KM (1978) Epidemiology of Diabetes and its vascular lesions. Elsevier, New York.
- Putnam J, Allshouse J, Scott Kantor L (2002) U.S. Per Capita Food Supply Trends: More Calories, Refined Carbohydrates, and Fats. Food Review 25: 2-15.
- McCrory MA, Suen VM, Roberts SB. Biobehavioral influences on energy intake and adult weight gain.J Nutr. 2002 Dec; 132: 3830S-3834S.
- Astrup A, Raben A (1995) Carbohydrate and obesity. Int J Obes 19 (Suppl 5): S27-S37
- Weststrate JA, van het Hof KH, van den Berg H, Velthuis-te-Wierik EJ, de Graaf C, Zimmermanns NJ, Westerterp KR, Westerterp-Plantenga MS, Verboeket-van de Venne WP (1998) A comparison of the effect of free access to reduced fat products or their full fat equivalents on food intake, body weight, blood lipids and fat-soluble antioxidant levels and haemostasis variable. Eur J Clin Nutr 52: 389-395
- Wing RR, Hill JO (2001) Successful weight loss maintenance. Annu Rev Nutr 21: 323-341
- Bortz WM, Wroldson A, Morris P, Issekutz B Jr. (1967) Fat, carbohydrate, salt and weight loss. Am J Clin Nutr 20: 1104-1112
- Bortz WM, Howat P, Holmes WL (1968) Fat, CHO, salt, and weight loss: further studies. Am J Clin Nutr 21: 1291-1301
- Golay A, Allaz AF, Morel Y, de Tonnac N, Tankova S, Reaven G (1996) Similar weight loss with low- or high-CHO diets. Am J Clin Nutr 63: 174-178
- Alford BB, Blankenship AC, Hagen RD (1990) The effects of variations in CHO, protein, and fat content of the diet upon weight loss, blood values, and nutrient intake of adult obese women. J Am Diet Assoc 90: 534-540
- Lean MEJ, Han TS, Prvan T, Richmond PR, Avenell A (1997) Weight loss with high and low carbohydrate 1200 kcal diets in free living women. Eur J Clin Nutr 51: 243-248
- Kreitzman SN, Coxon AY, Szaz KF (1992) Glycogen storage: illusions of easy weight loss, excessive weight regain, and distortions in estimates of body composition.Am J Clin Nutr 56 (1 Suppl): 292S-293S.
- Wyatt HR, Donahoo WT, Grunwald GK, Wing RR, Hill JO. Average steps per day for long-term weight loss in the National Weight Control Registry. Obes Res 2001; 9: 192s (Abstract)
- Blair SN (1993) Evidence for success of exercise in weight loss and control. Ann Intern Med 119: 702-706
- Tremblay A, Almeras N, Boer J, Kranenbarg EK, Despres JP (1994) Diet composition and postexercise energy balance. Am J Clin Nutr 59: 975-979
Figure 1. Daily weight loss on a low calorie diet with varying carbohydrate and fat content. Source: Bortz et al., 1967